DNA/cDNA/kDNA Knots
"Bursicon, the insect cuticle-hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2", by C-W. Luo, E. M. Dewey, S. Sudo, J. Ewer, S. Y. Hsu, H-W. Honegger, A. J. W. Hsueh, Proceedings of the National Academy of Sciences U.S., Feb. 22 2005, 102, 8, 2820 - 2825
Molting and ecdysis are regulated by six hormones. Bursicon acts last (after ecdysis completes), triggering melanization and sclerotization of the new cuticle (as well as wing expansion). LGR protein receptors (preserved in invertibrates and vertibrates) comes in three forms:
- Group A, LGR vertibrate
- Group B, DLGR2 Drosophila melanogaster and LGR4/5/6 vertibrates
- Group C, relaxin/INSL3 mammals
Bursicon has been identified with "burs" (a cysteine knot protein), has "pburs" as its heterodimer and is described in this paper. "pburs' is also a cysteine knot protein.
PCR cDNA amplification was used for both "burs" as well as "pburs":
- burs
- 5'- AAGGACACTCGCAGTCGGGCCGA -3'
- 5'- CTATTGCAGAGCAATGCGCCGGA -3'
- pburs
- 5'- TGGGAGTGGGTCAGCATGCA -3'
- 5'- TAGTTTATGTTGAGGCATTA -3'
![]()
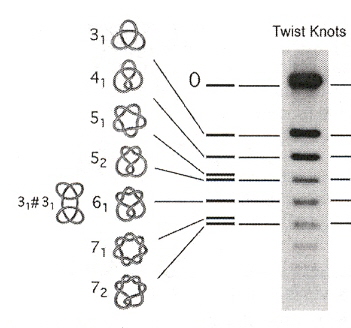
"DNA knots reveal a chiral organization of DNA in phage capsids", by J. Arsuaga, M. Vazquez, P. McGuirk, S. Trigueros, D. Sumners, J. Roca, Proceedings of the National Academy of Sciences of the USA, June 28 2005, 102, 26, 9165 - 9169
Experimental evidence of the knot structure found in DNA. Examples of knots are in the figure below.
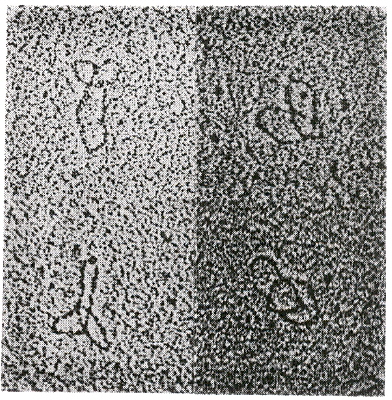
"A knotted free minicircle in kinetoplast DNA", by K. Ryan, T. A. Shapiro, C. A. Rauch, J. D. Griffith, P. T. Englund, Proceedings of the National Academy of Sciences U.S.A., Aug. 1988, 85, 16, 5844 - 5848
During replication of kDNA, minicircles are released from the network as individually covalently closed circles (under the influence of topoisomerase II). The free circles are replicated, reattaching to antipodes of the kinetoplast (thus doubling the network size) (see: Kinetoplast Replication) . During this process, trefoil knots of minicircles were found in Trypanosoma equiperdum. Different methods were used to detect these trefoil knots, a most dramatic one being electron microscopy.
Trefoil Knot Structure in Kinetoplast Minicircles
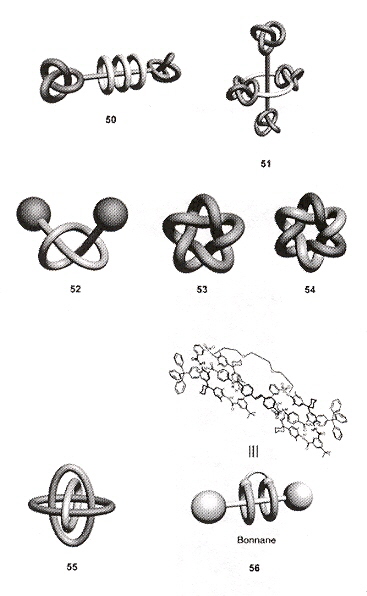
Protein Knots
The question of the existence of protein knotted structures is complicated. If proteins are viewed as a linear string of amino acids, then protein knots may not be said to exist. However, such a definition excludes complications including not only of: definitions, protein folding, disulfide bonds, metal coordination, hydrogen bonds, etc.a.
Knots are defined for closed paths. However, if line segments from each N and C terminus of a protein, extended to a sphere, and connected to a great circle on this enveloping sphereb, then "tightened"c or smoothed d and unraveled e. Might a knot not be found and thus defined (using an Alexander Polynomial or a Jones polynomial, for example)? Would such an operation be justified? It has been pointed out that the termini of proteins usually lie at or near the exterior of the globular conformation of proteins due to their being hydrophillic. In fact, if restricted to being within 1% of the exterior of the globular conformation, 96% of the proteins examined in the Brookhaven Protein Databank satisfy this definition b. It is then found that two proteins (2CAB and 9CA2) have the Alexander Polynomial A(t) = 1 - t + t2 (trefoil knot) b. This procedure has been defined in greater depth, using a statistical scoring method to define knots in proteins. Figure-of-eight protein knots have also been found c. It has ben postulated that during protein folding, a "swap" c of α-helix structures may take place, supporting a generalized knot-tying process a, f, but this can happen only at a specific time of folding while this swapping process can take place. In addition to knotted proteins, protein catenanes have been discovered g, h, and circular proteins (named circulins) have been reported! i where the 30 amino acids in are cyclized by peptide bonds. Finally, even rotaxanes have been found to be naturally occurring in proteins and peptides!
N and C Terminals
Smoothing
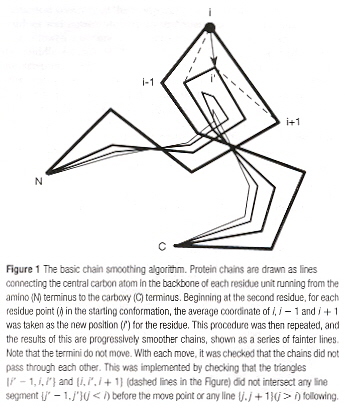
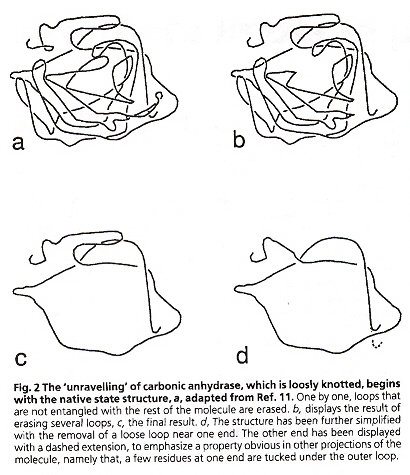
Unravelling
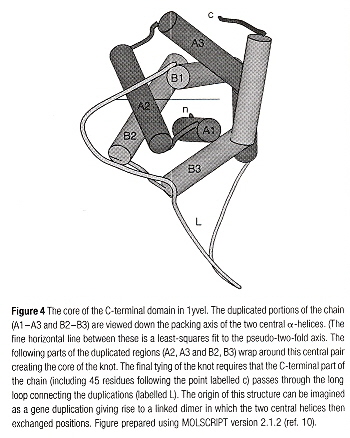
Swapping
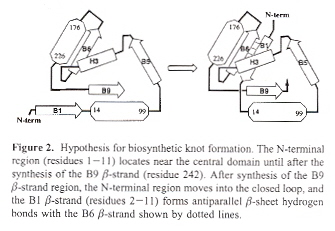
Time of Swapping
Protein Catenanes
a "A Real Knot in Protein", by F. Takasugawa, A. Kamitori, Journal of the American Chemical Society, 1996, 118, 37, 8945 - 8946
b "Fit to be tied", by M. L. Mansfield, Nature of Structural Biology, March 1997, 4, 3, 166 - 167
c "A tangled problem", by W. R. taylor, K. Lin, Nature, Jan. 2 2003, 421, 25
d "A deeply knotted protein structure and how it might fold", by W. R. Taylor, Nature, Aug. 24 2000, 406, 916 - 919
e "Are there knots in proteins?", by M. L. Mansfield, Structural Biology, April 1994, 1, 4, 213 - 214
f "Three-dimensional domain duplication, swapping and stealing", by J. Heringa, W. R. Taylor, Current Opinion in Structural Biology, 1997, 7, 3, 416 - 421
g "Knots in Proteins", by C. Liang, K. Mislow, Journal of the American Chemical Society, 1994, 116, 11189 - 11190
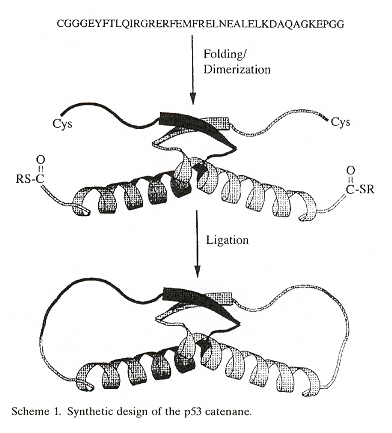
h "Design and Synthesis of a Protein Catenane", by L. Z. Yan, P. E. Dawson, Angewandte Chemie International Edition, 2001, 40, 19, 3625 - 3626
i "Circulins A and B: Novel HIV-Inhibitory Macrocyclic Peptides from the Tropical Tree Chassalia parvifolia", bt K. R. Gustafson, R. C. Sowder II, L. E. Henderson, I. C. Parsons, Y. Kashman, J. H. Cardellina II, James B. McMahon, R. W. Buckheit, L. K. Pannell, M. R. Boyd, Journal of the American Chemical Society, 1994, 116, 20, 9337 - 9338
j "Effect of Catenation pn Protein Dolding Stability", by H-Z. Zhou, Journal of the American Chemical Society, 2003, 125, 20, 9280 - 9281
"Folding Studies on a Knotted Protein", by A. L. Mallam, S. E. Jackson, Journal of Molecular Biology, March 11 2005, 346, 5, 1409 - 1421
Trefoil protein knots in which the knot is composed of few (ten) amino acids have been found. However, such knots are composed of ten amino-acid polypeptide, thus they barely make a knot (are named shallow trefoil knots). If only shallow protein knots exist, perhaps the data supporting the existence of protein-knots is questionable (ie: protein knots doen't really exist)? However, at least thirteen examples have been found with a deep trefoil or figure-of-eight knotted structure. Acetohydroxy acid isomeroreuctase has a figure-of-eight structure, with 200 amino acid derivatives on one side, 70 amino acid residues on the other side of the knot.
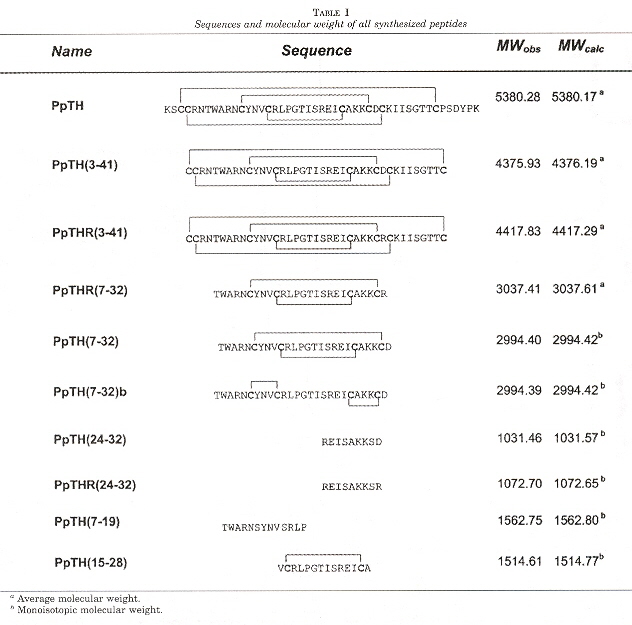
"Structural Dissection of a Highly Knotted Peptide Reveals Minimal Motif with Antimicrobial Activity", by M. Vila-Perelló, A. Sánchez-Vallet, F. García-Olmedo, A. Molina, D. Andreu, The Journal of Biological Chemistry, Jan. 14 2005, 280, 2, 1661 - 1668
Most antimicrobial peptides are linear and of eukaryotic origin. This paper focuses upon highly knotted, cysteine-rich plant thionin (PpTH). PpTH derivatives were synthesized to find a minimally complex peptide which still retained the bioactivity of thionin. It is believed that the antimicrobial peptides studied involve simple disruption of microbial plasma membranes, thus resistance would require substantial membrane redesign (and thus would be non-affordable).
Both knotted and unknotted PpTH derivatives were found to be significantly more active than PpTH on Gram-negative bacteria, but with comparable PpTH activity for Gram-positive bacteria.
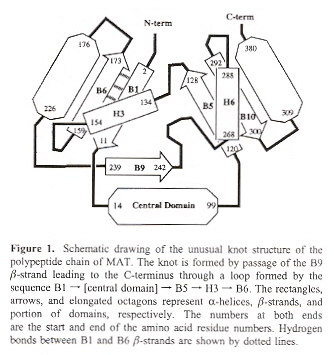
"Functional Assignment Based on Structural Analysis: Crystal Structure of the yggJ Protein (HI0303) of Haemophilus influenzae Reveals an RNA Methyltransferase With a Deep Trefoil Knot", by F. Forouhar, J. Shen, R. Xiao, T. B. Acton, G. T. Montelione, L. Tong, PROTEINS: Structure, Function, and Genetics, Nov. 1 2003, 53, 2, 329 - 332
A trefoil knot is reportedly found in the yggJ Protein (HI0303) of Haemophilus influenzae that is crucial to methyl transfer from S-adenosylmethionine to carbon, nitrogen, or oxygen in DNA, RNA, and proteins.
"Structure of the YibK Methyltransferase From Haemophilus influenzae (HI0766): A Cofactor Bound at a Site Formed by a Knot", by K. Lim, H. Zhang, A. Tempczyk, W. Krajewski, N. Bonander, J. Toedt, A. Howard, E. Eisenstein, O. Herzberg, PROTEINS: Structure, Function, and Genetics, April 1 2003, 51, 1, 56 - 67
HI0766 contains a knot that forms a binding crevace for cofactor S-adenosylhomocysteine. HI0766 are related to rRNA/tRNA methyltransferases.
"Kalata B8, a novel antiviral circular protein, exhibits conformational flexibility in the cystine knot motif", by N. L. Daly, R. J. Clark, M. R. Plan, D. J. Craik, The Biochemical Journal, Feb. 1 2006, 393, Part 3, 619 - 626
The Kalata B8 protein contains three pairs for a total of six cysteine amino acids. Each pair of cysteines are bound by a disulphide bridge a. As Kalata B8 is also cyclic (a member of the class of cyclotide cyclic proteins), this protein forms a knot, thus Kalata B8 is a cyclic knottin.
a "Seamless Proteins Tie Up Their Loose Ends", by D. J. Craik, Science, March 17 2006, 311, 5767, 1563 - 1564
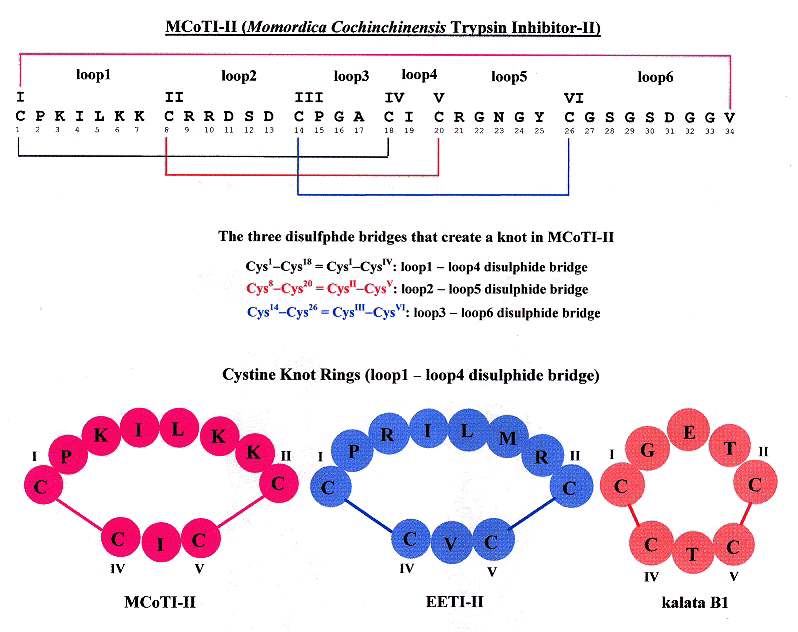
"Knots in Rings THE CIRCULAR KNOTTED PROTEIN MOMORDICA COCHINCHINENSIS TRYPSIN INHIBITOR-II FOLDS VIA A STABLE TWO-SULFIDE INTERMEDIATE", by M. Čemažar, N. L. Daly, S. Häggblad, K. P. Lo, E. Yalyaningsih, D. J. Craik, The Journal of Biological Chemistry, March 24 2006, 281, 12, 8224 - 8232
Circulins (circular proteins) that naturally fold with a knot structure due to disulfide bonds are examined. Specifically, Mommordica cochinchinensic trypsin inhibotor-II (or MCoTI-II), Ecballium elaterium (or EETI-II), and kalata B1 are compared. All three of these have a cyclic cystine knot (CCK) structure. The figure below helps illucidate this structure.
Cyclic Cysine Knots
"Rotaxanes of Cyclic Peptides", by V. Aucagne, D. A. Leigh, J. S. Lock, A. R. Thomson, Journal of the American Chemical Society, 2006, 128, 6, 1784 - 1785
Rotaxanes are reported to be synthesized from well-known cyclic peptides.
Back to TopChiral Chemistry & Knots
"Knotting and Threading of Molecules: Chemistry and Chirality of Molecular Knots and Their Assemblies", by O. Lukin, F. Vögtle, Angewandte Chemie International Edition, Feb. 25 2005, 44, 10, 1456 - 1471
A good summary of the subject by a leading expert. The authors suggest replacing the terms catenane and rotaxane by knotane.
Dendroknots
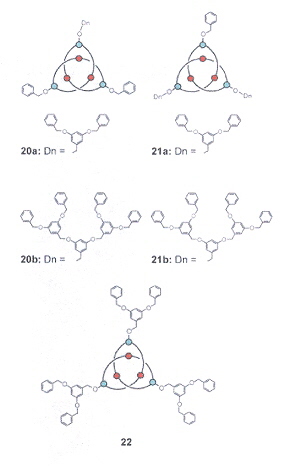
Hydroxyknotanes, Phosphorylated Knotanes, Sulfonated Knotanes
Complex Knotanes